How do parents respond to changing offspring energy demands throughout the breeding season?
LINKED PAPER
Parental resource allocation among offspring varies with increasing brood age in Black-legged Kittiwakes Rissa tridactyla. Robertson, G.S., Bolton, M. & Monaghan, P. 2015.
Bird Study. DOI: 10.1080/00063657.2015.1040370
Few studies have examined how food distribution varies among offspring throughout development and how this affects overall reproductive success. In this study, we examined how brood feeding rate and parental nest attendance changed as broods of Black-legged Kittiwakes developed, and how resource allocation between chicks varied with increasing brood age.
Parents need to make decisions about how to allocate limited resources among offspring within a single brood or litter. Theoretically, parents should invest more resources in offspring with the greatest need (Godfray et al. 1995) however larger offspring tend to be fed more often than their smaller siblings, even when they are not signalling the greatest need (Price et al. 1996). In bird species with asynchronously hatching young, parents preferentially feed older chicks, which are of higher reproductive value than younger chicks (Parker et al. 2002). This is because older chicks are usually larger and more competitive than other chicks in the brood, hence tend to receive food from adults at a higher rate. They are therefore able to sustain higher growth rates and are more likely to survive to fledging (Braun & Hunt 1983, Merkling et al. 2014).
Parental investment and resource allocation may be expected to vary over time as the benefits of parental care and offspring energy demand change (Tveraa et al. 1998). Parents match feeding rates to chick energy requirements, which are highest during the period of maximum growth and decline as chicks approach fledging (Emms & Verbeek 1991, Roby 1991). Hence, adults may decrease the amount of food delivered to chicks after energy demands decline or as a strategy to encourage chicks to leave the nest (Emms & Verbeek 1991, Roby 1991). Parents may also visit the nest less often during late chick-rearing and devote less time to attending the nest as chicks become larger and less vulnerable to predation (Chiaradia & Kerry 1999).
Timing of maximum growth and peak energy demand vary among chicks of different hatching order (Mock & Schwagmeyer 1990). The reproductive value of offspring increases with age (Redondo & Carranza 1989), hence parents may be expected to increase the proportion of resources allocated to younger chicks in a brood later in the developmental period (Shizuka & Lyon 2009).
In our study, we expected:
- first-hatched chicks to be fed more frequently and achieve higher growth rates than younger chicks
- parental feeding and nest attendance to decline after chicks reached maximum growth
- parents to increase the proportion of food allocated to younger chicks with increasing brood age
The study was carried out on Coquet Island, northeast England (55° 20’ N, 1° 32’ W) during the Kittiwake chick-rearing period from June – July 2012 (Figure 1). Thirty randomly-selected Kittiwake nests with broods of two were observed from hatching until fledging, and the hatching order of chicks was recorded for each brood. First-hatched (A) chicks were marked to avoid confusion with second-hatched (B) chicks. For each nest, we recorded whether or not an adult was attending the nest when its partner returned with food, which chick was fed during feeding bouts, how many times an adult regurgitated food to each chick and the order in which chicks were fed. Growth rate was calculated for each chick.

Contrary to what we expected, we found no significant difference in feeding rates or growth rates of A and B chicks. This finding may have been a result of the extremely high food availability in the area surrounding the colony in 2012.
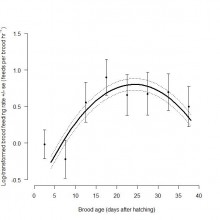
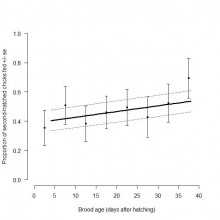
Throughout the chick-rearing period, both adult nest attendance and number of prey deliveries made per brood declined with increasing brood age. Brood feeding rate increased until chicks were 21 – 25 days old, before declining as chicks approached fledging age at 30 – 40 days old (Figure 2). Parental resource allocation also varied between chicks in a brood as brood age increased. The probability of B chicks receiving a higher proportion of total feeds delivered to a brood increased with brood age (Figure 3). This may be expected given that younger chicks become more competitive and more valuable to parents as they get older. This may explain why overall feeding and growth rates of A and B chicks were similar in this study.
Figure 2. Relationship between log-transformed feeding rate per brood (feeds delivered per brood hr-1) and brood age (days after hatching). Mean ± standard error was calculated for each five-day brood age category
Figure 3. Relationship between proportion of second-hatched chicks fed and brood age (days after hatching). Mean proportion of occasions where second-hatched chicks were fed ± standard error was calculated for each five-day brood age category
Our results emphasise the importance of considering how parents allocate resources among offspring throughout the rearing period for understanding the effect of parental investment on offspring survival in bird species with asynchronously hatching young.
References
Braun, B. M. & Hunt, G.L. 1983. Brood reduction in Black-legged Kittiwakes. Auk 100: 469–476. View
Chiaradia, A.F. & Kerry, K.R. 1999. Daily nest attendance and breeding performance in the Little Penguin Eudyptula minor at Phillip Island, Australia. Mar. Ornithol. 27: 13–20. View
Emms, S. K. & Verbeek, N.A. 1991. Brood size, food provisioning and chick growth in the Pigeon Guillemot Cepphus columba. Condor 93: 943–951. View
Godfray, H.C.J. 1995. Signaling of need between parents and young: parent-offspring conflict and sibling rivalry. Am. Nat. 146: 1–24. View
Merkling, T., Agdere, L., Albert, E., Durieux, R., Hatch, S.A., Danchin, E. & Blanchard, P. 2014. Is natural hatching asynchrony optimal? An experimental investigation of sibling competition patterns in a facultatively siblicidal seabird. Behav. Ecol. Sociobiol. 68: 309–319. View
Mock, D.W. & Schwagmeyer, P.L. 1990. The peak load reduction hypothesis for avian hatching asynchrony. Evol. Ecol. 4: 249–260. View
Parker, G.A., Royle, N.J. & Hartley, I.R. 2002. Intrafamilial conflict and parental investment: a synthesis. Proc. R. Soc. B 357: 295–307. View
Price, K., Harvey, H. & Ydenberg, R. 1996. Begging tactics of nestling Yellow-headed Blackbirds, Xanthocephalus xanthocephalus, in relation to need. Anim. Behav. 51: 421–435. View
Redondo, T. & Carranza, J. 1989. Offspring reproductive value and nest defence in the magpie (Pica pica). Behav. Ecol. Sociobiol. 25: 369–378. View
Roby, D.D. 1991. Diet and postnatal energetic in convergent taxa of plankton-feeding seabirds. Auk 108: 131–146. View
Shizuka, D. & Lyon, B.E. 2009. Family dynamics through time: brood reduction followed by parental compensation with aggression and favouritism. Ecol. Lett. 16: 315–322. View
Tveraa, T., Sether, B.E., Aanes, R. & Erikstad, K.E. 1998. Regulation of food provisioning in the Antarctic Petrel; the importance of parental body condition and chick body mass. J. Anim. Ecol. 67: 699–704. View
Image credit
Featured image: Kittiwakes © Gail S. Robertson
If you want to write about your research in #theBOUblog, then please see here.