LINKED PAPER
Applying global criteria to tracking data to define important areas for marine conservation. Lascelles, B., Taylor, P., Miller, M., Dias, M.P., Oppel, S., Torres, L., Hedd, A., le Corre, M., Phillips, R.A., Scott, S., Weimerskirch, H. & Small, C. 2016 (in press). Diversity and Distributions.
 Of the 359 species of seabirds, 159 (44%) are classified as globally threatened (i.e. Vulnerable, Endangered or Critically Endangered; IUCN 2015). This places this group amongst one of the most threatened of all birds (BirdLife International 2013). Pelagic species are particularly at risk – many populations of albatrosses, petrels and penguins have faced medium to severe declines over the last decades (Croxall et al. 2012). Two main threats have driven these declines: incidental bycatch in fisheries and the predation by introduced mammals at the colonies (usually islands; Žydelis et al. 2009, Anderson et al. 2011, Croxall et al. 2012). While the impact of the latter threat is relatively easy to quantify (and, to a certain extent, to address; e.g. Norton & Warburton 2014), identifying the magnitude and occurrence of at-sea mortality is far more challenging. Until very recently, we knew very little about the foraging ecology of most species – we simply didn’t know where the birds were going to find food – so how could we quantify, and then tackle, their main at-sea threats?
Of the 359 species of seabirds, 159 (44%) are classified as globally threatened (i.e. Vulnerable, Endangered or Critically Endangered; IUCN 2015). This places this group amongst one of the most threatened of all birds (BirdLife International 2013). Pelagic species are particularly at risk – many populations of albatrosses, petrels and penguins have faced medium to severe declines over the last decades (Croxall et al. 2012). Two main threats have driven these declines: incidental bycatch in fisheries and the predation by introduced mammals at the colonies (usually islands; Žydelis et al. 2009, Anderson et al. 2011, Croxall et al. 2012). While the impact of the latter threat is relatively easy to quantify (and, to a certain extent, to address; e.g. Norton & Warburton 2014), identifying the magnitude and occurrence of at-sea mortality is far more challenging. Until very recently, we knew very little about the foraging ecology of most species – we simply didn’t know where the birds were going to find food – so how could we quantify, and then tackle, their main at-sea threats?
In the last three decades the scientific community has witnessed an incredible advance in animal tracking technologies, and the consequent increase in the number of studies focusing on seabird foraging behaviours and migratory movements. Since the early 90s, when some pioneering studies appeared (e.g Jouventin & Weimerskirch 1990), hundreds of papers reporting tracking data have been published, and it’s now clearly a dominant topic in seabird research. For example, at the 2nd World Seabird Conference held in Cape Town, South Africa (November 2015), ca. 30 % of the studies presented involved seabird tracking.

These studies have proved relevant to tackle the above mentioned conservation problems, and in 2004 BirdLife International created, the Tracking Ocean Wanderers – The Global Procellariiform Tracking Database. The aim was to establish a centralised database that could be used to understand the movements of albatrosses and petrels, identify areas where overlap with fishing effort was highest and help reducing their mortality due to by-catch (BirdLife International 2004). In an unprecedented response, scientists worldwide joined the initiative and contributed their datasets.
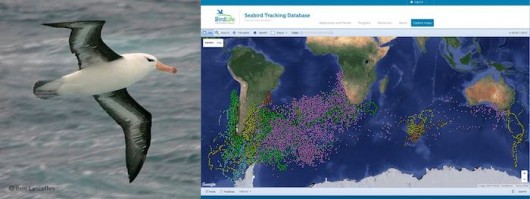
Ten years later, the database has been expanded to cover all seabird species – and is now a preeminent example of cooperation between scientists and conservationists. More than 150 researchers, from 22 countries, contribute their datasets. It now holds over 5 million data points for 85 species (plus 20 not yet shown online), with information in all ocean basins (Fig 3). Researchers maintain full control over their data; they can opt to have their data visible online (with or without zoom limitations), or to simply show a metadata table. And, most importantly, they are always consulted regarding any potential use of the data, either by BirdLife International or by any third party that might be interested in a scientific collaboration.
What do we do with these data?
Tracking data have proved to be a fundamental tool in seabird conservation (Burger & Shaffer 2008). Understanding the distribution patterns of individuals and populations is often the first step needed when attempting to address a certain threat. For example, identifying the overlap between the major world fishing fleets and albatross distribution was crucial to address the by-catch problem, and reduce it in some areas by more than 95% (Maree et al. 2014). Along with at sea-surveys, tracking studies are the most important source of data to map the at-sea range of seabird species, an essential layer of information in any Marine Spatial Planning exercise, or when identifying Marine Protected Areas (Lascelles et al. 2014).
However, analysing data collected from individual birds, and scaling that up to the population level, is a challenging exercise (e.g. Gutowsky et al. 2015). Birds from the same colony can choose distinctive sites to forage (between-individuals flexibility; e.g. Patrick et al. 2013), and even different individuals within a population can vary in the degree of faithfulness to their foraging sites (within-individuals flexibility; Dias et al. 2011, Wakefield et al. 2015). Recent evidence points to an extreme variability among species in this “flexible-consistent” axis of their foraging behaviour (Weimerskirch 2007, Wakefield et al. 2015), and sometimes we can find such variability even between different populations of the same species (e.g. in Black-browed albatrosses; Wakefield et al. 2011, Catry et al. 2013). To understand this in practical conservation terms, we need to assess medium to long term consistency in site use at the population level, case by case, and during different stages of the breeding cycle (e.g. Robertson et al. 2014). This requires long term studies (covering several years), at multiple colonies, which are unfortunately still missing in most cases. It also demands a collaborative effort to pool data – reinforcing, again, the utility of having a single repository of data collected by different research teams.
In an attempt to address these questions and boost the utilization of tracking data for conservation, BirdLife International, with the support of the scientific community, developed a unique tool to identify foraging hotspots from bird’s individual locations (Lascelles et al. 2016). The tool is a combination of R scripts that can be applied to any dataset of seabird tracking, which characterises potential Important Bird and Biodiversity Areas (IBA – Fishpool & Evans 2001). It includes an objective approach to define species-specific smoothing parameters (h values) for kernel density estimation based on area-restricted search behaviour (Fauchald and Tveraa 2003), and an analysis to determine whether sites identified from tracked individuals are also representative for the wider population. The application of this approach to more than 60 species and 252 datasets (i.e., unique combinations of data collected for a certain species, in a given colony and breeding stage) resulted in the identification of more than 1000 IBAs (which can be seen at https://maps.birdlife.org/marineIBAs/default.html; Fig. 4). The total area of the IBAs identified during this analysis amounts to 4.3% of the world’s oceans, the future protection of these sites would make a significant contribution to achieving Aichi target 11, which required the designation of 10% of the world’s oceans as MPAs by 2020 (SCBD 2014).
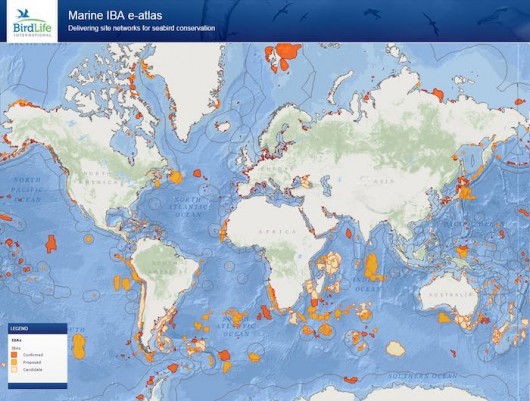
With over 50% of the species assessed listed as Globally Threatened by IUCN, this network of IBAs, is of key important for marine conservation efforts. Together these sites show where species can be most effectively conserved as a group and where potential threats may have population level impacts. Best practice management of activities that negatively affect seabirds in these areas, such as through the designation of Marine Protected Areas, would make a vital contribution to the conservation of seabirds (and other marine life found in these areas) and prove once more that science-based conservation is the way forward to halt and reverse the declines many species have undergone in recent decades.
References
Anderson, O.R.J., Small, C., Croxall, J.P., Dunn, E.K., Sullivan, B.J., Yates, O. & Black, A. 2011. Global seabird bycatch in longline fisheries. Endangered Species Research 14: 91–106. View
BirdLife International. 2004. Tracking ocean wanderers: the global distribution of albatrosses and petrels. Results from the Global Procellariiform Tracking Workshop, 1–5 September, 2003, Gordon’s Bay, South Africa. Cambridge, UK: BirdLife International. View
BirdLife International. 2013. State of the world’s birds: indicators for our changing world. Cambridge, UK: BirdLife International. View
Burger, A. & Shaffer, S.A. 2008. Application of Tracking and Data-Logging Technology in Research and Conservation of Seabirds. The Auk 125: 253-264. View
Catry, P., Lemos, R., Brickle, P., Phillips, R.A., Matias, R. & Granadeiro, J.P. 2013. Predicting the distribution of a threatened albatross: the importance of competition, fisheries and annual variability. Progress in Oceanography 110: 1-10. View
Croxall, J.P., Butchart, S.H.M., Lascelles, B., Stattersfield, A.J., Sullivan, B., Symes, A. & Taylor, P. 2012. Seabird conservation status, threats and priority actions: a global assessment. Bird Conservation International 22: 1-34. View
Dias, M.P, Granadeiro, J.P., Phillips, R.A., Alonso, H. & Catry, P. 2011. Breaking the routine: individual Cory’s shearwaters shift winter destinations between hemispheres and across ocean basins. Proceedings of the Royal Society B 278: 1786-1793. View
Fauchald, P. & Tveraa, T. 2003. Using First-Passage Time in the Analysis of Area-Restricted Search and Habitat Selection. Ecology 84: 282-288. View
Fishpool, L.D.C. & Evans, M.I. 2001 Important Bird Areas in Africa and associated islands: priority sites for conservation. Newbury and Cambridge, UK: Pisces Publications and BirdLife International. BirdLife Conservation Series No. 11.
Granadeiro, J.P., Phillips, R.A., Brickle. P. & Catry, P. 2011. Albatrosses following fishing vessels: how badly hooked are they on an easy meal? PLoS ONE 6: e17467. View
Gutowsky, S.E., Leonard, M.L., Conners, M.G., Shaffer, S.A. & Jonsen, I.D. 2015. Individual-level variation and higher-level interpretations of space use in wide-ranging species: an albatross case study of sampling effects. Frontiers in Marine Science 2:93. View
IUCN. 2015. The IUCN Red List of Threatened Species. Version 2015-4. https://www.iucnredlist.org. Downloaded on 12 December 2015.
Jouventin, P. & Weimerskirch, H. 1990. Satellite tracking of Wandering albatrosses. Nature 343, 746 – 748. View
Lascelles, B., Sciara, G.N.D., Agardy, T., Cuttelod, A., Eckert, S., Glowka, L., Hoyt, E., Llewellyn, F., Louzao, M., Ridoux, V. & Tetley, M.J. 2014. Migratory marine species: their status, threats and conservation management needs. Aquatic Conservation: Marine and Freshwater Ecosystems: 24: 111–127. View
Lascelles, B., Taylor, P., Miller, M., Dias, M.P., Oppel, S., Torres, L., Hedd, A., le Corre, M., Phillips, R.A., Scott, S., Weimerskirch, H. & Small, C. 2016. Applying global criteria to tracking data to define important areas for marine conservation. Diversity and Distributions. In press
Maree, B.A., Wanless, R.M., Fairweather, T.P., Sullivan, B.J. & Yates, O. 2014. Significant reductions in mortality of threatened seabirds in a South African trawl fishery. Animal Conservation 17: 520–529. View
Norton, D. & Warburton, B. 2014. The potential for biodiversity offsetting to fund effective invasive species control. Conservation Biology 29: 5–11. View
Patrick, S.C., Bearhop, S., Grémillet, D., Lescroël, A., Grecian, W.J., Bodey, T.W., Hamer, K.C., Wakefield, E., Le Nuz, M. & Votier, S.C. 2013. Individual differences in searching behaviour and spatial foraging consistency in a central place marine predator. Oikos 123: 33-40. View
Robertson, G.S., Bolton, M., Grecian, W.J. & Monaghan, P. 2014. Inter‑ and intra‑year variation in foraging areas of breeding kittiwakes (Rissa tridactyla). Marine Biology 161:1973–1986. View
SCBD. 2014. Global Biodiversity Outlook 4. Montréal, Canada, 155 pages. View
Wakefield, E.W., Phillips, R.Q., Trathan, P.N., Arata, J., Gales, R., Huin, N., Robertson, G., Waugh, S.M., Weimerskirch, H. & Matthiopoulos, J. 2011. Habitat preference, accessibility, and competition limit the global distribution of breeding Black-browed Albatrosses. Ecological Monographs 81: 141–167. View
Wakefield, E.D., Cleasby, I.R., Bearhop, S., Bodey, T.W., Davies, R.D.D, Miller, P.I., Newton, J., Votier, S.C. & Hamer, K.C. 2015. Long-term individual foraging site fidelity—why some gannets don’t change their spots. Ecology 96:3058–3074. View
Weimerskirch, H. 2007. Are foraging for unpredictable resources? Deep-Sea Research II 54: 211–223. View
Žydelis, R., Bellebaum, J., Österblom, H., Vetemaa, M., Schirmeister, B., Stipniece, A., Dagys, M., van Eerden, M. & Garthe, S. 2009. Bycatch in gillnet fisheries – An overlooked threat to waterbird populations. Biological Conservation 142: 1269–1281. View
Image credit</strong >
Featured image: Black-browed Albatross Thalassarche melanophris © Ben Lascelles.




