A rare mineral in eggshells acts as a shock absorber
LINKED PAPER
A rare mineral, vaterite, acts as a shock absorber in the eggshell of a communally nesting bird. Portugal, S.J., Bowen, J. & Riehl, C. 2017. IBIS. DOI: 10.1111/ibi.12527. VIEW
Birds’ eggshells are mostly composed of calcite, a ubiquitous form of calcium carbonate that is also found in seashells and limestone. Layered on top of the calcite shell is a thin, waxy coating known as the cuticle, which repels water from the surface of the egg and prevents microbes from invading the shell’s pores. In some species of birds, the shell cuticle is even more complex, coated with tiny nanospheres of a mineral called vaterite.
Vaterite was first identified in avian eggshells in the late 1970’s, but its presence has always been puzzling (Board and Perrott 1979). Like calcite, vaterite is a form of calcium carbonate (CaCO3); the only difference is in the crystal structure. But unlike calcite, vaterite is extremely rare in nature. It’s only found in a few odd places – gallstones and fish otoliths, for example – and its crystal structure wasn’t even described until 2013 (Kabalah-Amitai et al. 2013). Its apparent rarity may be due to its poor thermodynamic stability; whereas calcite has been called “nature’s ceramic,” vaterite has a higher solubility and tends to transform to the more stable calcite unless the conditions are just right. So what is vaterite doing in birds’ eggshells?
In the communally breeding cuckoos of South and Central America, the vaterite coating on the eggshell is particularly elaborate – and particularly mystifying. This lineage of cuckoos has an unusual breeding system in which several females lay eggs in the same nest, sharing incubation and parental care duties with their fellow group members. In Guira Cuckoos, Guira guira, the white, chalky vaterite coating is overlaid on the blue calcite shell like a lace doily on a table. In the anis, Crotophaga spp., the white coating forms a thin layer on top of the blue calcite shell like the bloom on a plum. This white coating is fresh and bright when the egg is first laid, but over the course of incubation it becomes scratched and abraded, revealing the blue shell beneath (Fig. 1).
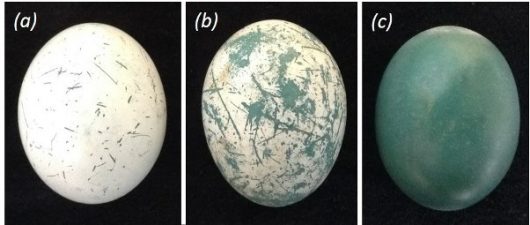
In a previous study, I found that anis use the appearance of the vaterite coating as a built-in “freshness indicator:” freshly laid eggs are white, older eggs are blueish. This allows anis to gauge the synchrony of the shared clutch (since eggs laid at the same time are about the same color) and to remove eggs that were laid out-of-synch – a useful ability for a communal breeder (Riehl 2010). But I wondered whether vaterite might have another function as well. Back in 1979, when vaterite was first identified on the surface of ani eggshells, it was proposed to have a structural function – by regulating gas exchange by blocking pores in the eggshell, for example, or by strengthening the shell so it would be less likely to crack (Board and Perrott 1979).
My colleagues Steve Portugal, James Bowen, and I set out to test these hypotheses using eggshells of Greater Anis, Crotophaga major, from my long-term study population in Panama. To test the hypothesis that the vaterite coating might affect gas exchange across the eggshell during embryonic development, I used a clever experimental set-up designed by Steve. Instead of trying to measure rates of gas exchange in entire eggs, I cut small fragments of the shell and glued them to plastic Eppendorf tubes filled with water. By weighing the shell-plus-tube units over the course of several days in a desiccator, I was able to compare the rate of water loss in shell fragments with the vaterite coating to fragments from the same shells without the vaterite coating. Surprisingly, there was no difference: the presence of vaterite didn’t have an effect on how rapidly water vapour was conducted through the pores of the eggshell.
Steve and I decided that we needed to test the second hypothesis: that the vaterite coating might reduce the risk of shell fracture during incubation. We already suspected that ani eggshells might need to be unusually strong, since clutch sizes are very large – with up to four females laying in one nest, each clutch can contain up to 20 eggs (Fig. 2)! Large clutches mean that the eggs roll and knock against each other every time the incubating adult moves the eggs around in the nest (a necessary behaviour that prevents the embryo from sticking to the eggshell and ensures that each egg gets the right amount of heat during incubation).

We enlisted James, a specialist in materials engineering, to further investigate the physical properties of vaterite. His scanning electron microscope (SEM) images showed that the vaterite coating – which looks like a solid layer to the naked eye – is actually composed of piles of tiny spheres just a few microns in diameter (Fig. 3). The calcite shell alone was unusually thick (about 300 microns, on average), and the layer of vaterite spherules increased this even more (by another 20 microns). Furthermore, sophisticated measurements of the physical properties of vaterite (using a fancy machine called a nanoindenter) revealed that the hardness and elasticity of vaterite is pretty similar to that of calcite. Using those data, James calculated the expected impact of two ani eggs colliding (as might happen when eggs are turned in the nest, for example). He found that the depth of surface deformation caused by the contact should only be about 1 micron, which is much less than the thickness of the vaterite coating. These results are consistent with the hypothesis that the vaterite spheres are crushed or compressed on contact, dampening the shock of the impact and essentially acting as a “shock absorber” – just as originally proposed back in 1979.

There’s one more possibility, though, that we didn’t test: the hypothesis that the vaterite spherules also help prevent bacteria from adhering to the surface of the eggshell. Elegant work on eggs of other bird species has found that the presence of cuticular nanospheres reduces the odds that the egg will become infected by pathogens (D’Alba et al. 2016). This work has mostly been done in species where the nanospheres are made of calcium phosphate, not vaterite, but it’s possible that vaterite spherules do the same thing.
References
Board, R.G. & Perrott, H.R. 1979. Vaterite, a constituent of the eggshells of the non-parasitic cuckoos, Guira guira and Crotophagi ani. Calc. Tissue Intl. 29: 63–69. VIEW
D’Alba, L., Maia, R., Hauber, M.E. & Shawkey, M.D. 2016. The evolution of eggshell cuticle in relation to nesting ecology. Proc. R. Soc. B 283: 20160687. VIEW
Kabalah-Amitai, L., Mayzel, B., Kauffmann, Y., Fitch, A.N., Bloch, L., Gilbert, P.U.P.A. & Bokroy, B. 2013. Vaterite crystals contain two interspersed crystal structures. Science 340: 454–457. VIEW
Riehl, C. 2010. A simple rule reduces costs of extragroup parasitism in a communally breeding bird. Curr. Biol. 20: 1830–1833. VIEW
Image credit
Featured image: Greater Ani, Crotophaga major © Christina Riehl
If you want to write about your research in #theBOUblog, then please see here.





